A product that was able to reduce incapacity for work during respiratory diseases. A modified version targeting viral disease at the time of the COVID-19 pandemic.
IMUNOCOMPLEX 2020

IMUNOCOMPLEX 2020
IMUNOCOMPLEX 2020:
- It was developed primarily for long-term training of mucosal immunity.
- The site of infection is the mucous membranes, which is the body’s natural barrier in contact with the outside world within the respiratory, digestive and urogenital tracts and eyes.
- Modulation of exaggerated inflammation is important from civilazition diseases point of view. Excessive inflammation damages our body even in the severe course of COVID-19.
- By sucking IMUNOCOMPLEX 2020 (preferably left under the tongue as long as possible), we systematically train non-specific immunity for a long time. The condition of mucous membranes will affect whether we get sick and how seriously.
- By reducing the viral load (washing or disinfecting hands, using chirurgical masks and respirators), and long-term training of mucosal immunity, we will improve our individual chances.
IMUNOCOMPLEX 2020 contains:
Monascus purpureus NEXARS
With the current hygienic epidemiological situation associated with SARS CoV-2 infection and the availability of a number of 3D structures relevant to this infection in the Protein Data Bank, it is possible to perform a blind docking study of many of the proteins involved in the infection. To this end, several small molecules that were previously used in research projects are available in the laboratory.
The term “blind docking” means that nothing is assumed about the binding site of any ligand to the target protein, so docking is performed using the entire available surface of the protein. Docking studies for the following small molecule secondary metabolites (pigments) of Monascus purpureus NEXARS are described below: Ankanaflavin (afl), monascin (ma), monacolin K (mk) a monascuspiloin (mp).
From the chemical structure of the compounds, coordinate files in pdbqt format are generated using the molview.org web server (copyright Herman Bergwerf) followed by AutoDockTools from the Vina suite to anchor protein targets.
The results of these blind docking studies are presented for 4 protein targets whose 3D structures are present in the PDB. An orthorhombic box (grid size 1 Å) with a sufficient “cushion” around the protein is used for each protein. Blind docking is performed using the Autodock VINA software suite (Trott, O. & Olson, A.J. 2010, J. Comput. Chem. 31, 455-461).
| Compound | SARS CoV-2 main protease (PDB ID 6YB7, resolution 1,25Á) | SARS CoV-2 papain-like protease (trimeric form,PDB ID 6W9C, resolution 2,7Á) | SARS CoV-2 NSP15 endoribonucleosis(monomer,PDB ID 6W01, resolution 1,9Á) | Human ACE2 (virus-recognized receptor, PBD ID 1R42, resolution 2,2Á) |
| Ankanaflavin | -8,7 | -7,1 | -7,0 | -7,9 |
| Monascin | -8,3 | -7,8 | -6,6 | -8,0 |
| Monakolin K | -8,4 | -8,4 | -7,7 | -7,7 |
| Monascuspiloin | -8,5 | -7,9 | -6,0 | -8,0 |
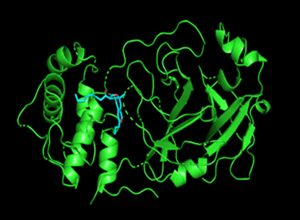
SARS CoV2 main protease (green) with the location of the anchored Ankaflavin (afl).
SARS CoV2 papain protease trimer (blue) SARS CoV2 papain protease trimer with anchored molecule of monacolin K (green) near the central zinc ion (grey sphere)
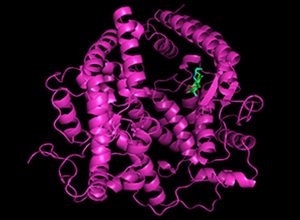
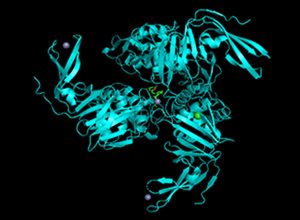
Human protein ACE2 (purple, it is a receptor for viral entry into the cell) showing the dock location of two small molecules Monascin (ma, in green) and Monascuspiloin (mp, in green) in the receptor cavity. Note that ma and mp are structurally very similar.
Probiotic strains
In order to properly tune the immune system and maintain its vigilance, we used 4 of our new original probiotic strains of human origin in the preparation. This is important for mucosal immunomodulation of exaggerated inflammation, which is one of the causes of an exaggerated reaction of the immune system, which can lead to a serious course and long-term consequences. According to our results in the mouse model, the desired systemic switching of the Th1 Th2 response occurs.
The selected 4 probiotic strains are not commonly used probiotic bacteria of animal origin, but are of human origin from our own database. To ensure safety, we performed an analysis of all genes of the new microorganism and thus ruled out the possibility of transmitting antibiotic resistance. See e.g. Streptococcus salivarius GH NEXARS – – an original biotechnological production strain internationally protected under the Patent Procedure under the Budapest Treaty and published the entire genome in the GenBank database.
GenBank is part of the International Nucleotide Sequence Database Collaboration, which includes DNA DataBank of Japan (DDBJ), the European Nucleotide Archive (ENA), and GenBank at NCBI. The admission process is reviewed. The recorded genome acquires a unique international identification.
We enhance our probiotics with colostrum – the first thing a mother gives to her child and serves as an immunonutrient support in a new world full of microbes.
We also studied the genes of our original probiotic bacteria on the primary and secondary metabolic pathways of bile acid biosynthesis, and based on the results, there is a presumption for a decrease in cholesterol after administration of our original human probiotics, which we verify, among other things, in the ongoing placebo-controlled study. The possibility of reducing the civilization diseases and modulating inflammation increase the overall defenses.
Our good probiotic bacteria are alive in pills.
Polyphenols – natural substances
In our preparations we use exclusively natural dyes which not only stain the product, but especially contain polyphenols, which not only protect our genetic information from damage (we measure DNA repair damage using Comet assay) but also have the potential according to docking studies to link to COVID-19 and thereby reducing its effects.
In addition, we have preliminary results of curcumin modification (native form it is practically insoluble in aqueous media), which is dissolved in aqueous media resulting in an increase its biodistribution and thus an increase in its migrastatic and anti-inflammatory effect depending on its biodistribution / availability.

Our water soluble formulation

Native form
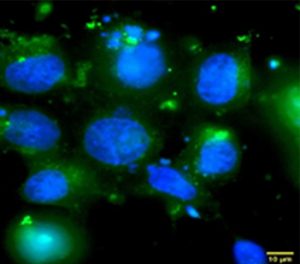
Intracellular distribution of curcumin (green) in FaDu cells after 48 hours of incubation using a QPHASE multimodal holographic microscope. Cell nuclei were stained with Hoechst 33342 (blue). Scale 10 μm, 100x mag. Curcumin concentration was determined as the IC25 value (10.5 μM).
Bioformulation
We process raw materials in an original way, ie. we formulate them in a pill that are bioavailable to the human body. For example, bioformulations containing natural polyphenols based on curcumin are used (native form practically insoluble in aqueous media), which is formulated into aqueous media and increases its biodistribution and thus its increase in migrastatic and anti-inflammatory effect depending on its biodistribution / availability.